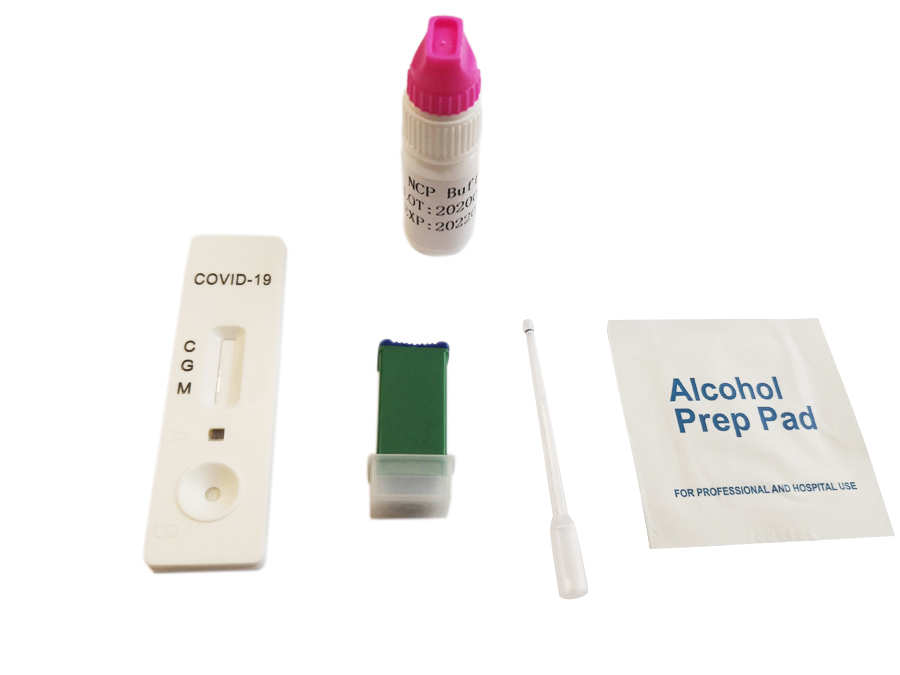
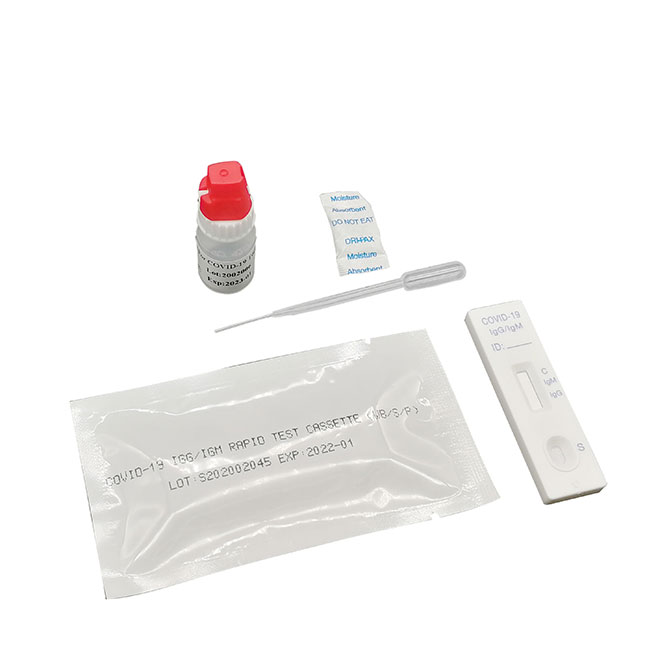
Quick Details
Detection kit for Covid-19
Packaging & Delivery
| Packaging detail:Standard export package Delivery detail:within 7-10 workdays after receipt of payment |
Specifications
[INTENDED USE]
The AMRDT100 IgG/IgM Rapid Test Cassette is a lateral flow chromatographic immunoassay for the qualitative detection of antibodies (IgG and IgM) to Novel coronavirus in human Whole Blood/Serum/Plasma.
It provides an aid in the diagnosis of infection with Novel coronavirus.

[SUMMARY]
Early January 2020, a novel coronavirus (SARS-CoV-2, formerly known as 2019-nCoV) was identified as the infectious agent causing an outbreak of viral pneumonia in Wuhan, China, where the first cases had their symptom onset in December 2019.
Coronaviruses are enveloped RNA viruses that are distributed broadly among humans, other mammals, and birds and that cause respiratory, enteric, hepatic, and neurologic diseases.Six coronavirus species are known to cause human disease. Four viruses-229E, OC43, NL63, and HKU1 are prevalent and typically cause common cold symptoms in immunocompetent individuals. The two other strains severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome coronavirus (MERS-CoV) are zoonotic in origin and have been linked to sometimes fatal illness.
Coronaviruses are zoonotic, meaning they are transmitted between animals and people. Common signs of infection include respiratory symptoms,fever, cough, shortness of breath and breathing difficulties. In more severe cases, infection can cause pneumonia, severe acute respiratory syndrome, kidney failure and even death.
Standard recommendations to prevent infection spread include regular hand washing, covering mouth and nose when coughing and sneezing, thoroughly cooking meat and eggs. Avoid close contact with anyone showing symptoms of respiratory illness such as coughing and sneezing.
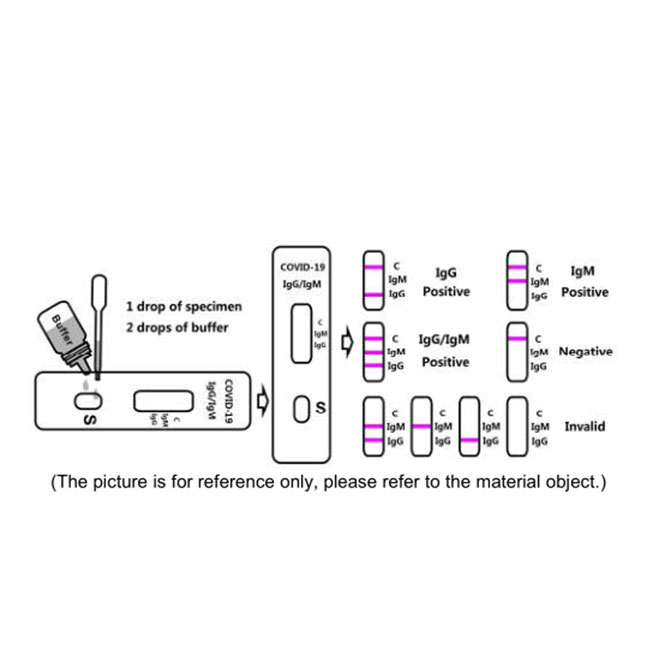
[PRINCIPLE]
The AMRDT100IgG/IgM Rapid Test Cassette is a qualitative membrane strip based immunoassay for the detection of antibodies (IgG and IgM) to Novel coronavirus in human Whole Blood/Serum/Plasma. The test cassette consists of: 1) a burgundy colored conjugate pad containing Novel coronavirus recombinant envelope antigens conjugated with Colloid gold (Novel coronavirus conjugates), 2) a nitrocellulose membrane strip containing two test lines (IgG and IgM lines) and a control line (C line). The IgM line is pre-coated with the Mouse anti-Human IgM antibody, IgG line is coated with Mouse anti-Human IgG antibody. When an adequate volume of test specimen is dispensed into the sample well of the test cassette, the specimen migrates by capillary action across the cassette. IgM anti-Novel coronavirus, if present in the specimen, will bind to the Novel coronavirus conjugates. The immunocomplex is then captured by the reagent pre-coated on the IgM band, forming a burgundy colored IgM line, indicating a Novel coronavirus IgM positive test result. IgG anti-Novel coronavirus if present in the specimen will bind to the Novel coronavirus conjugates. The immunocomplex is then captured by the reagent coated on the IgG line, forming a burgundy colored IgG line, indicating a Novel coronavirus IgG positive test result. Absence of any T lines (IgG and IgM) suggests a negative result. To serve as a procedural control, a colored line will always appear at the control line region indicating that proper volume of specimen has been added and membrane wicking has occurred.

[WARNINGS AND PRECAUTIONS]
For healthcare professionals and professionals at point of care sites.
Do not use after the expiration date.
Please read all the information in this leaflet before performing the test.
The test cassette should remain in the sealed pouch until use.
All specimens should be considered potentially hazardous and handled in the same manner as an infectious agent.
The used test cassette should be discarded according to federal, state and local regulations.
[COMPOSITION]
The test contains a membrane strip coated with Mouse anti-Human IgM antibody and Mouse anti-Human IgG antibody on the test line, and a dye pad which contains colloidal gold coupled with Novel coronavirus recombinant antigen.
The quantity of tests was printed on the labeling.
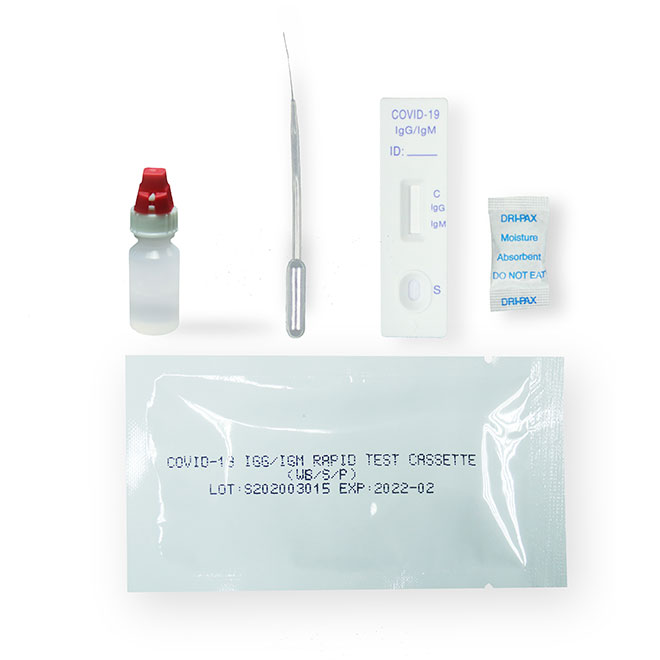
Materials Provided
Test cassettePackage insert
Buffer
Materials Required But Not Provided
Specimen collection containerTimer
[STORAGE AND STABILITY]
Store as packaged in the sealed pouch at the temperature (4-30℃ or 40-86℉). The kit is stable within the expiration date printed on the labeling.
Once open the pouch, the test should be used within one hour. Prolonged exposure to hot and humid environment will cause product deterioration.
The LOT and the expiration date were printed on the labeling.

[SPECIMEN]
The test can be used to test Whole Blood/Serum/Plasma specimens.
To collect whole blood, serum or plasma specimens following regular clinical laboratory procedures.
Separate serum or plasma from blood as soon as possible to avoid hemolysis. Use only clear non-hemolyzed specimens.
Store specimens at 2-8℃ (36-46℉) if not tested immediately. Store specimens at 2-8℃ up to 7 days. The specimens should be frozen at
-20℃ (-4℉) for longer storage. Do not freeze whole blood specimens.
Avoid multiple freeze-thaw cycles. Prior to testing, bring frozen specimens to room temperature slowly and mix gently. Specimens containing visible particulate matter should be clarified by centrifugation before testing.
Do not use samples demonstrating gross lipemia, gross hemolysis or turbidity in order to avoid interference on result interpretation.

[TEST PROCEDURE]
Allow the test device and specimens to equilibrate to temperature (15-30℃or 59-86℉) prior to testing.
1.Remove the test cassette from the sealed pouch.
2.Hold the dropper vertically and transfer 1 drop of specimen to the specimen well(S) of the test device, then add 2 drops of buffer (approximately 70μl) and start the timer. See the illustration below.
3.Wait for colored lines to appear. Interpret the test results in 15 minutes. Do not read results after 20 minutes.
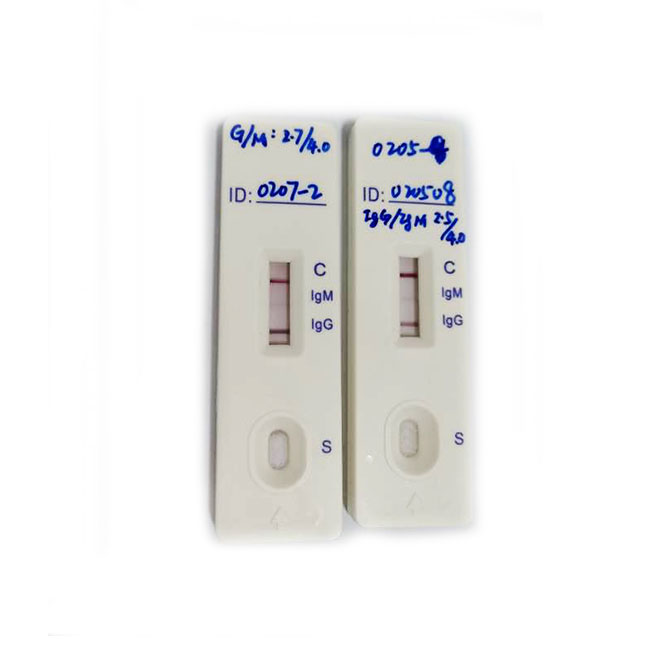
[INTERPRETATION OF RESULTS]
Positive: Control line and at least one test line appear on the membrane. The appearance of IgG test line indicates the presence of Novel coronavirus specific IgG antibodies. The appearance of IgM test line indicates the presence of Novel coronavirus specific IgM antibodies. And if both IgG and IgM line appear, it indicates that the presence of both Novel coronavirus specific IgG and IgM antibodies.
Negative: One colored line appears in the control region(C).No apparent colored line appears in the test line region.
Invalid: Control line fails to appear. Insufficient specimen volume or incorrect procedural techniques are the most likely reasons for control line failure. Review the procedure and repeat the test with a new test cassette. If the problem persists, discontinue using the test kit immediately and contact your local distributor.
[QUALITY CONTROL]
A procedural control is included in the test. A colored line appearing in the control region (C) is considered an internal procedural control. It confirms sufficient specimen volume, adequate membrane wicking and correct procedural technique.
Control standards are not supplied with this kit. However, it is recommended that positive and negative controls be tested as good laboratory practice to confirm the test procedure and to verify proper test performance.
[LIMITATIONS]
The AMRDT100 IgG/IgM Rapid Test Cassette is limited to provide a qualitative detection. The intensity of the test line does not necessarily correlate to the concentration of the antibody in the blood.
The results obtained from this test are intended to be an aid in diagnosis only. Each physician must interpret the results in conjunction with the patient’s history, physical findings, and other diagnostic procedures.
A negative test result indicates that antibodies to Novel coronavirus are either not present or at levels undetectable by the test.

[PERFORMANCE CHARACTERISTICS]
Accuracy
A side-by-side comparison was conducted using the Novel coronavirus IgG/IgM Rapid Test and a leading commercial PCR. 120 clinical specimens from Professional Point of Care site were evaluated. The following results are tabulated from these clinical studies:
A statistical comparison was made between the results yielding a sensitivity of 90.00%, a specificity of 97.78% and an accuracy of 95.83%.
Cross-Reactivity and Interference
1.Other common causative agents of infectious diseases were evaluated for cross reactivity with the test. Some positive specimens of other common infectious diseases were spiked into the Novel coronavirus positive and negative specimens and tested separately. No cross reactivity was observed with specimens from patients infected with HIV, HAV, HBsAg, HCV, HTLV, CMV, FLUA, FLUB, RSV and TP.
2.Potentially cross-reactive endogenous substances including common serum components, such as lipids, hemoglobin, bilirubin, were spiked at high concentrations into the Novel coronavirus positive and negative specimens and tested, separately. No cross reactivity or interference was observed to the device.
3.Some other common biological analytes were spiked into the Novel coronavirus positive and negative specimens and tested separately. No significant interference was observed at the levels listed in the table below.
Reproducibility
Reproducibility studies were performed for Novel coronavirus IgG/IgM Rapid Test at three physician office laboratories (POL). Sixty (60) clinical serum specimens, 20 negative, 20 borderline positive and 20 positive, were used in this study. Each specimen was run in triplicate for three days at each POL. The intra-assay agreements were 100%. The inter-site agreement was 100 %.