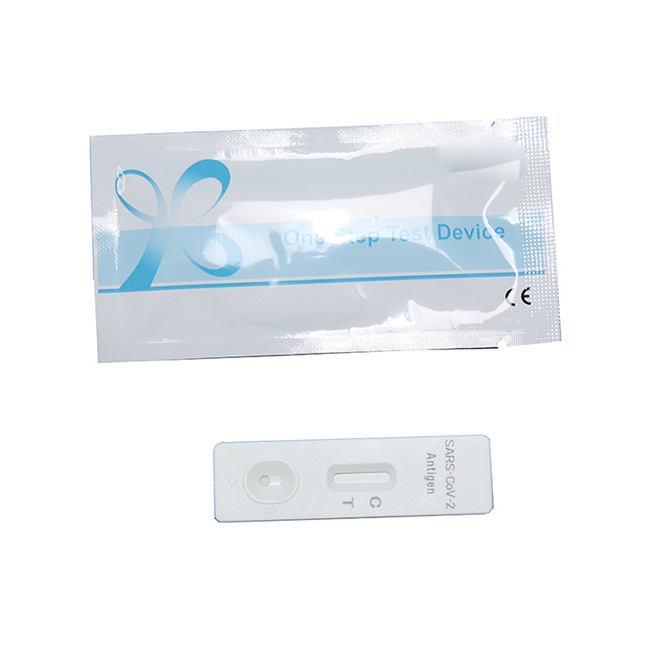
Early detection of SARS-CoV-2 infection
The test result is available in 10-15 min
Simple operation and highly- eficient test
Authoritative Antigen rapid test kit AMDNA10

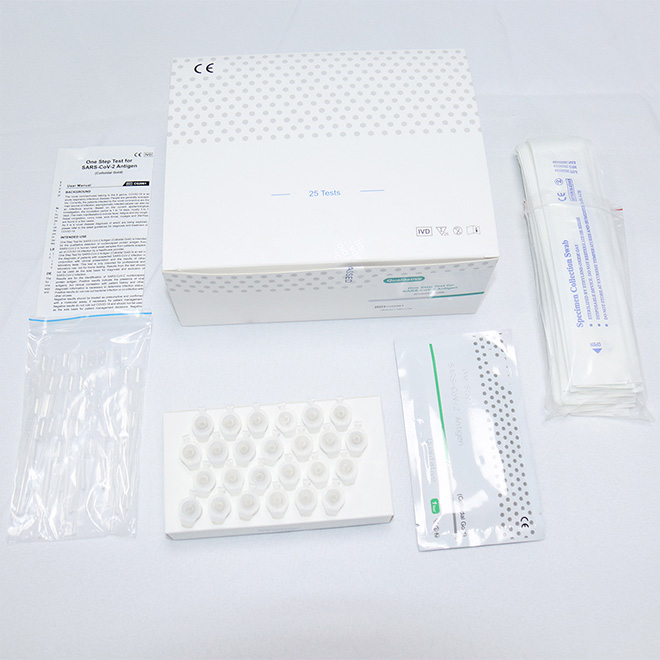
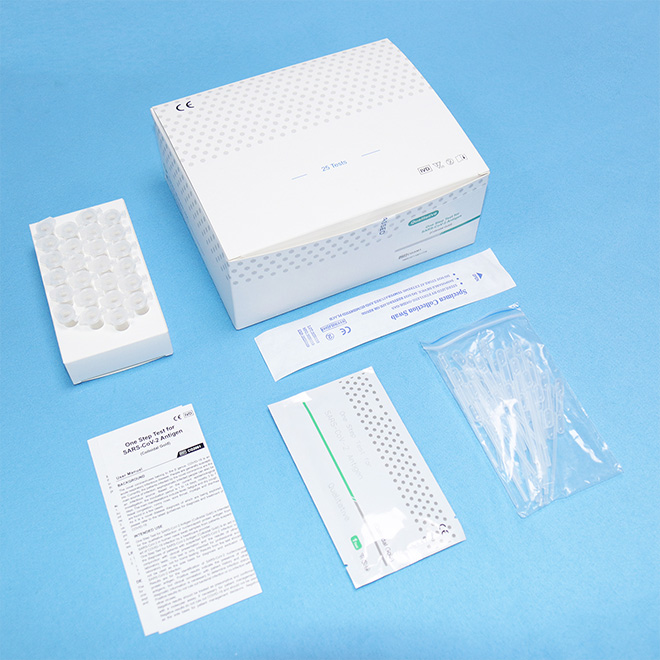
Authoritative Antigen rapid test kit AMDNA10 Purpose
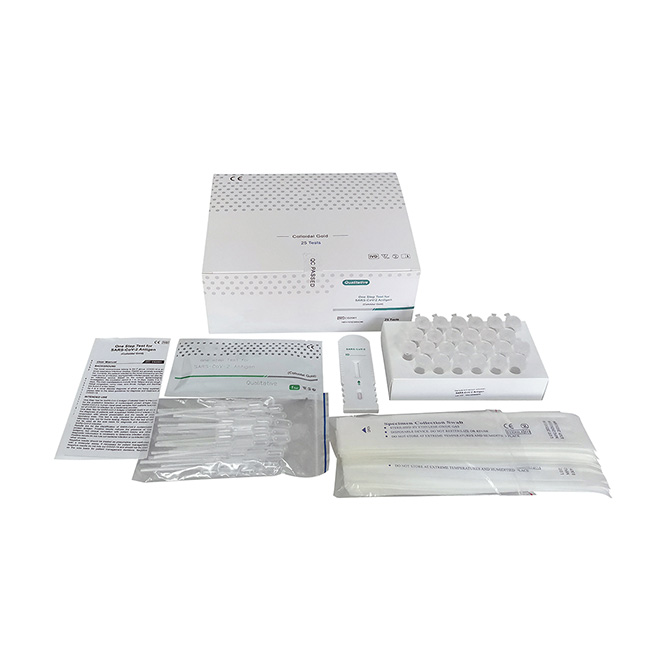
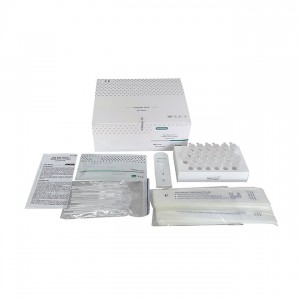
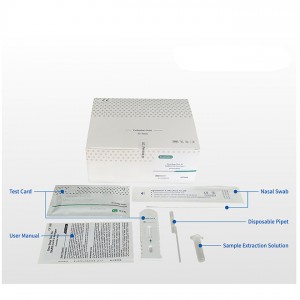
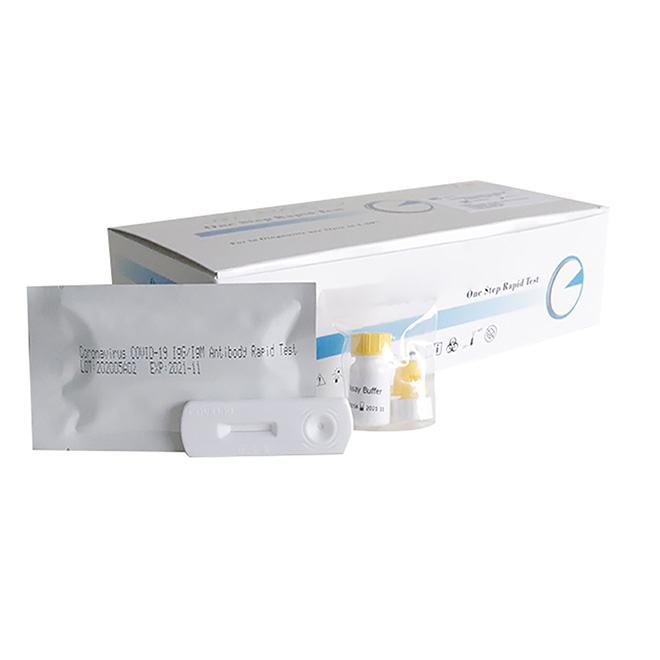
One Step Test for SARS-CoV-2 Antigen (Colloidal Gold) was developed by Getein Biotech, Inc., which was intended for the qualitative detection of 2019-Novel Coronavirus antigen in human nasal swab samples from patients suspected of COVID-19 infection.
The aim of the clinical agreement study was to compare and evaluate the clinical performance of One Step Test for SARS-CoV-2 Antigen (Colloidal Gold) with an RT-PCR test. The studies were performed at three sites in China from March to May 2020.
Authoritative Antigen rapid test kit AMDNA10 Experimental Materials
2.1 Trial reagent
Name: One Step Test for SARS-CoV-2 Antigen (Colloidal Gold)
Specification: 25 tests per box
Lot no.: GSC20002S (manufacturing date: March 4th, 2020)
Manufacturer: Getein Biotech, Inc.
Authoritative Antigen rapid test kit AMDNA10
2.2 Comparator reagent
Name: Real-Time Fluorescent RT-PCR Kit for Detecting SARS-CoV-2
Specifications: 50 reactions per kit
Manufacturer: BGI Genomics Co. Ltd.
PCR System: ABI 7500 Fast Real-Time PCR System with software v2.0.6
Viral RNA extraction kit: QIAamp Viral RNA Mini Kit (cat. #52904)