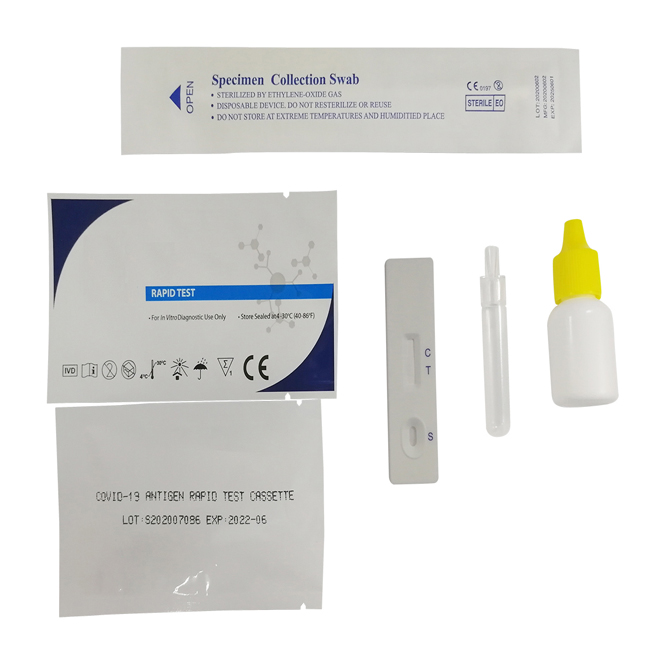
Quick Details
Specimens:
The detect specimens include nasopharyngeal swab and oropharyngeal swab.
Sample preparation can take according to the operation steps.
1.Specimen extraction reagent
2.Leave the swab in the reagent tube for one minute.
3.Pinch the extraction tube with fingers.
4.Insert a nozzle.
Packaging & Delivery
| Packaging detail:Standard export package Delivery detail:within 7-10 workdays after receipt of payment |
Specifications
COVID-19 Antigen Rapid Test Cassette AMRDT106:
SARS-CoV-2 Nucleocapsid Protein Detection:
Nucleocapsid (N) protein is the most abundant protein with highly conserved in SARS-CoV-2.
N protein is used as the core raw material of rapid diagnostic reagent forimmunology in the market.
COVID-19 Antigen Rapid Test Cassette Developed by Clongene:
Clongene has developed the COVID-19Antigen Rapid Test Cassette.The colloidal gold immunoassay
(CGIA) to detect nucleocapsid protein of SARS-CoV-2 is based on the principle of the double antibody-sandwich technique.
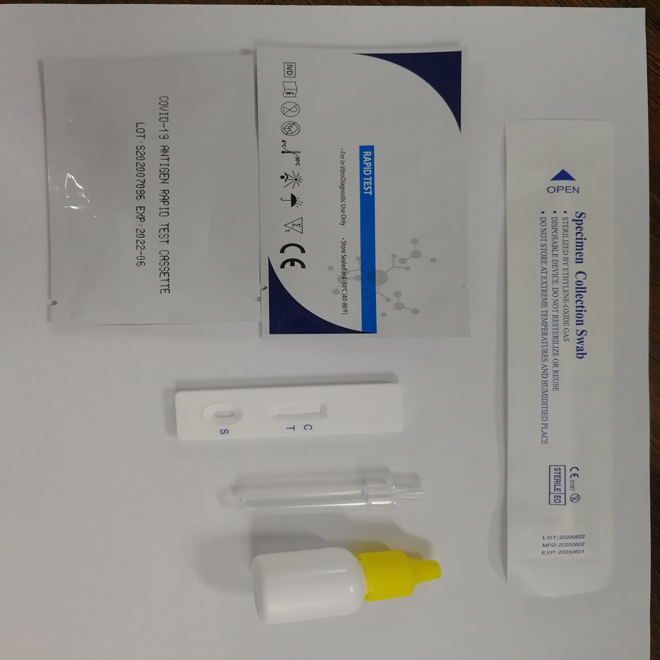
INTENDED USE:
The COVID-19 Antigen Rapid Test Cassette is a lateral flow immunoassay intended for the qualitative detection SARS-CoV-2 nucleocapsid antigens in nasopharyngeal swab and oropharyngeal swab from individuals who are suspected of CoVID-19 by their healthcare provider.Results are for the identification of SARS-CoV-2 nucleocapsid antigen.Antigen is generally detectable in nasopharyngeal swab and oropharyngeal swab during the acute phase of infection.Positive results indicate the presence of viral antigens, but clinical correlation with patient history and other diagnostic information is necessary to determine infection status.Positive results do not rule out bacterial infection or co-infection with other viruses.The agent detected may not be the definite cause of disease.Negative results do not rule out SARS-CoV-2 infection and should not be used as the sole basis for treatment or patient management decisions,including infection control decisions.Negative results should be considered in the context of a patient's recent exposures,history and the presence of clinical signs and symptoms consistent with COVID-19, and confirmed with a molecular assay, if necessary for patient management.The CoVID-19 Antigen Rapid Test Cassette is intended for use by trained clinical laboratory personnel specifically instructed and trained in vitro diagnostic procedures.
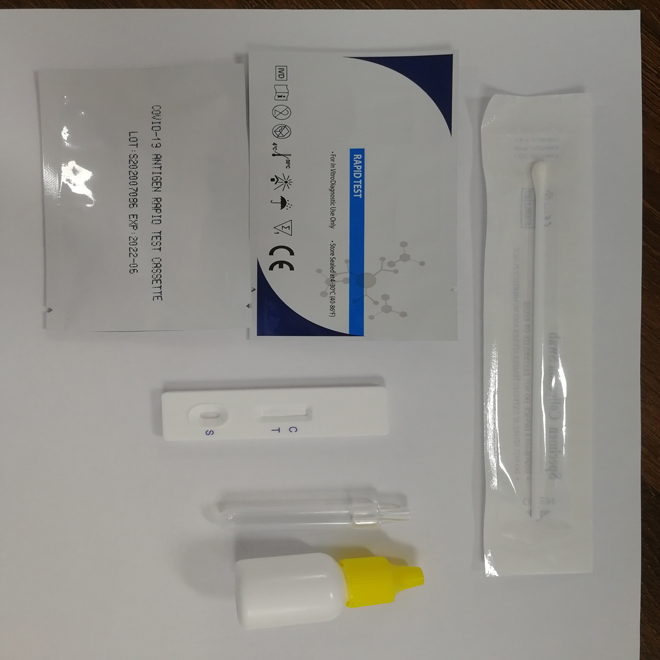
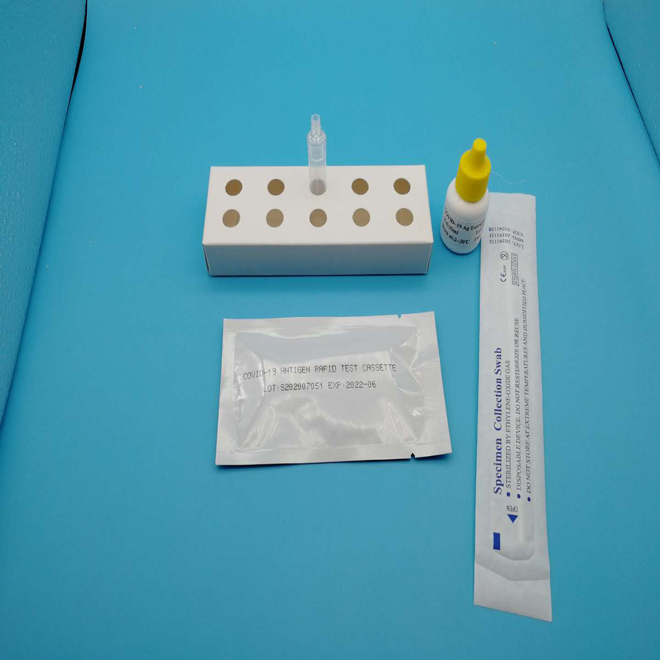
Specimens:
The detect specimens include nasopharyngeal swab and oropharyngeal swab.
Sample preparation can take according to the operation steps.
1.Specimen extraction reagent
2.Leave the swab in the reagent tube for one minute.
3.Pinch the extraction tube with fingers.
4.Insert a nozzle.
COMPOSITION:
The test cassette contains a membrane strip coated with anti-SARS-CoV-2 nuclenocapsid protein monoclonal antibody on the T test line,and a dye pad which contains colloidal gold coupled with SARS-CoV-2 nuclenocapsid protein monoclonal antibody.