Velox Details
Specimen generum: Saliva
Testis tempus: 15 minutes
Sensus: 98.10%
Specification:> 99.33%
Packaging & Delivery
| Packaging detail: Latin export sarcina Partus detail: in 7-10 fes post recepcionem solucionis |
Specifications
Medical COVID-19 Antigen Test Kit AMDNA12
Medica COVID-19 Antigen Saliva Test Kit AMDNA12 adhibetur pro detectione qualitativa coronavirus novae (COVID-19) antigenus in sample salivae, solum pro usu diagnostico in vitro.

Antigenus Test Kit CVID-19 adhibetur pro detectione qualitativa novae coronavirus (COVID-19) antigenus in specimen salivae, solum in usu vitro diagnostico.

Nova coronavirorum ad β genus pertinent.COVID-19 morbus infectious respiratorii acutus.Populus fere suscipit.In statu, aegri novo coronaviro infecti praecipue sunt infectionis fons;asymptomatici homines infecti possunt etiam fons infectiosus esse.
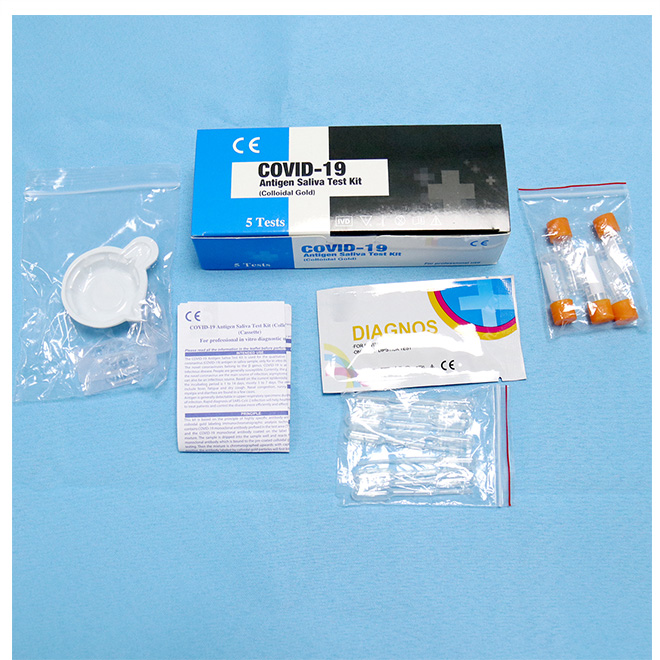
Medical COVID-19 Antigen Saliva Test Kit AMDNA12
Ex hodierna investigatione epidemiologica, incubatio periodus 1 ad 14 dies est, plerumque 3 ad 7 dies.Praecipua manifestationes comprehendunt febrem, lassitudinem et tussim aridam.Nasi congestio, liquescens nasus, faucium, myalgia et diarrhoea in paucis inveniuntur.Antigenum plerumque detectibile est in speciminibus respiratoriis superioribus in acuta periodo infectionis.

Celeri diagnosis SARS-CoV-2 infectio adiuvabit professionales sanitatis ad aegros curandos et morbum efficacius et efficacius moderandum.
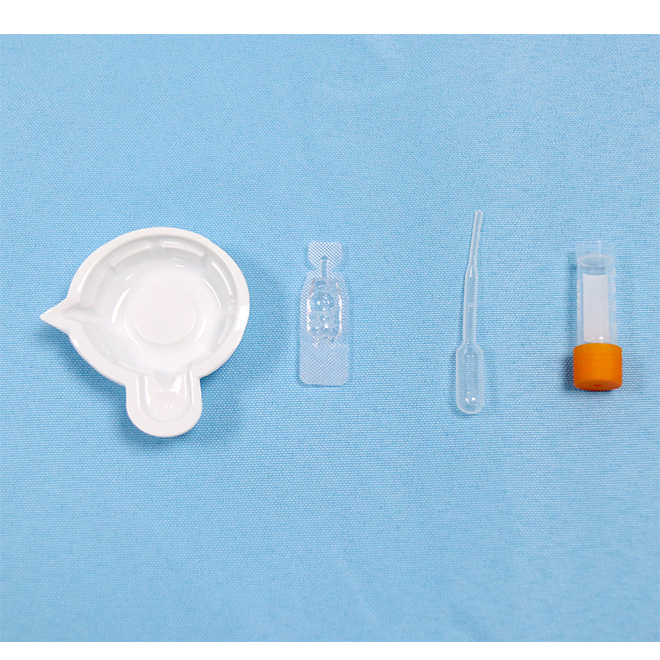
Medical COVID-19 Antigen Saliva Test Kit AMDNA12 fundatur in principio valde specifica anticorporis-antigenii reactionis et colloidalis auri technologiae technologiae immunochromatographicae.Reagens continet anticorpum COVID-19 monoclonale in area testi praefixam (T) membranae et anticorpus covid-19 monoclonale obductis pittacium pad-colloidalis auri mixtum.

Medical COVID-19 Antigen Saliva Test Kit AMDNA12
Specimen in specimen bene destillatur et cum anticorpore monoclonali covido-19 reflectitur quod ligatur particulis auri colloidalibus pre-coclatis cum tentantis.Tum mixtura sursum chromatographa cum effectibus capillaribus.Si positivus, anticorpus a particulis aureis colloidalibus intitulatum primum alligabit virus covid-19 in sample in chromatographia.Tunc conjugates ligantur anticorpore monoclonali COVID-19 in membrana fixa, et linea rubra apparet in area (T).Si negativa est, nulla linea rubra est in area experimenti (T).Num specimen COVID-19 antigenum contineat necne, linea rubra apparebit in qualitate regio (C).

Linea rubra apparens in regione qualitatis (C) est norma diiudicandi num satis exempla sint et num processus chromatographicus normale sit, et etiam pro regenti imperium internum servet.
Medical COVID-19 Antigen Saliva Test Kit AMDNA12 Features:
Specimen generum: Saliva
Testis tempus: 15 minutes
Sensus: 98.10%
Specification:> 99.33%

Components Medical COVID-19 Antigen Saliva Test Kit AMDNA12 habena in cassette:
Sample codex: sales et purgat buffered.
Codex pittacii: mus anti-COVID-19 monoclonalis anticorporis auri-intitulatum continet.Membrana nitrocellulosa;
Regio imperium: Capram anti-mus continet anticorpus IgG polyclonale et quiddam.Area test: mus anti-COVID-19 anticorpus monoclonale et quiddam continet.Codex absorbetur: charta valde bibula facta.