Quick Details
25 Sterile, single use specimen collection swabs
25 Single use extraction tubes with integrated dispensing tip
Each pouch contains: 1 test cassette and 1 desiccant
Packaging & Delivery
| Packaging detail:Standard export package Delivery detail:within 7-10 workdays after receipt of payment |
Specifications
Professional Antigen Rapid Test Kit AMDNA07
This product is used for the qualitative testing of new coronavirus SARS-CoV-2 IgM antibodies in human throat swab.

COVID-19 Antigen Rapid Test Kit is a solid phase immunochromatographic assay for the in vitro qualitative detection of antigen to 2019 Novel Coronavirus in human nasopharyngeal secretion or oropharyngeal secretion. This test kit provides only a preliminary test result for COVID-19 infection as a clinically-assisted diagnosis. The test kit is applicable to clinical system, medical institutions and scientific research field.
The novel Coronaviruses belong to the β genus.COVID-19 is an acute respiratory infectious disease. Currently, the patients infected by novel coronavirus are the main source of infection, asymptomatic infected people can also be an infectious source.
Based on the current epidemiological investigation, the incubation period is 1 to 14 days. The main manifestation includes fever, fatigue and dry cough. Nasal congestion, runny nose, sore throat, myalgia and diarrhea are found in a few cases. Coronavirus are enveloped RNA viruses that are distributed broadly among humans, other mammals, and birds and that cause respiratory, enteric, hepatic, and neurologic diseases.
Seven Coronavirus species are known to cause human disease. Four viruses – 229E, OC43, NL63, and HKU1 – are prevalent and typically cause common cold symptoms in immunocompetent individuals. The three other strains – severe acute respiratory syndrome Coronavirus (SARS-CoV), Middle East respiratory syndrome Coronavirus (MERS-CoV) and 2019 Novel Coronavirus (COVID-19) – are zoonotic in origin and have been linked to sometimes fatal illness. The COVID-19 Antigen Rapid Test Kit can detect pathogen antigens directly from nasopharyngeal swab or oropharyngeal swab specimens.

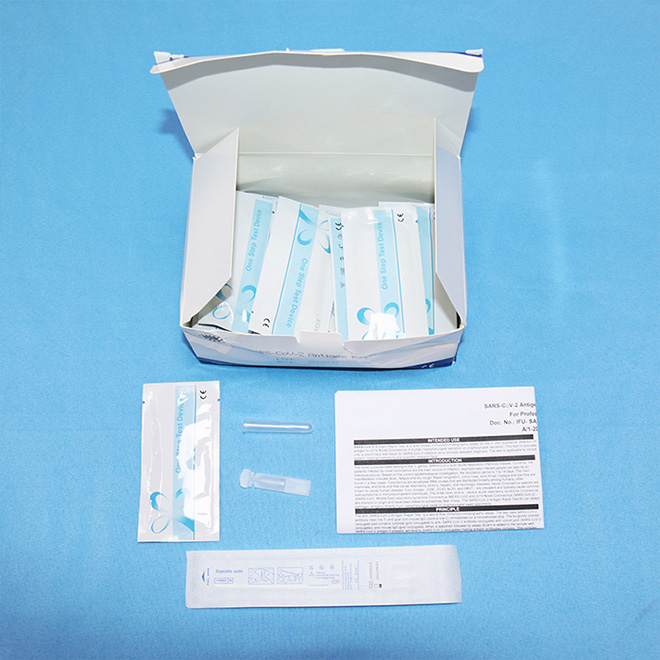
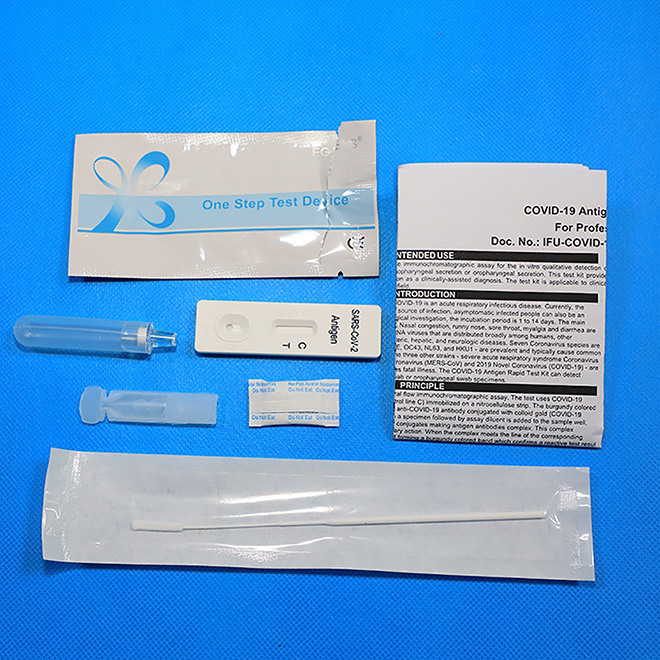
Professional Antigen Rapid Test Kit AMDNA07 Each box contains:
25 Novel Coronavirus (SARS-Cov-2) Antigen Rapid Test Kits 25 buffers
25 sterile, single use specimen collection swabs
25 single use extraction tubes with integrated dispensing tip
1 Instructions for Use (IFU).
Each pouch contains: 1 test cassette and 1 desiccant.

The anti-COVID-19 Antigen Rapid Test Kit is a lateral flow immunochromatographic assay. The test uses COVID-19 antibody (test line T) and goat anti-mouse IgG (control line C) immobilized on a nitrocellulose strip. The burgundy colored conjugate pad contains colloidal gold conjugated to anti-COVID-19 antibody conjugated with colloid gold (COVID-19 conjugates) and mouse IgG-gold conjugates. When a specimen followed by assay diluent is added to the sample well, COVID-19 antigen if present, will bind to COVID-19 conjugates making antigen antibodies complex. This complex migrates through nitrocellulose membrane by capillary action. When the complex meets the line of the corresponding immobilized antibody, the complex will be combined forming a burgundy colored band which confirms a reactive test result. Absence of a colored band in the test region indicates a non-reactive test result.
The test contains an internal control (C band) which should exhibit a burgundy colored band of the immunocomplex goat anti mouse IgG/mouse IgG-gold conjugate regardless of the color development on any of the test bands. Otherwise, the test result is invalid and the specimen must be retested with another device.